How To Draw Reaction Coordinate Diagram
Catalyst coordinate principle Reaction coordinate question diagrams enthalpy chemistry energy activation reactants arrow represents ea diagram exothermic below profile endothermic which barrier would How to interpret thermodynamics of reactions
How to Interpret Thermodynamics of Reactions
Solved: chapter 5 problem 10e solution Reaction coordinate 04.02 reaction coordinate diagrams and stability trends
Coordinate label
Reaction coordinate stabilityDiagram reaction catalyzed energy coordinate uncatalyzed vs potential label rate enzyme reactions chemistry diagrams kinetics pe catalyst catalysis following showing Reaction coordinate diagramSolved:draw a reaction coordinate diagram for a two-step reaction in.
Transition intermediate coordinateReaction coordinate diagram Reaction coordinate diagramsReaction coordinate diagrams energy rates chapter diagram ppt powerpoint presentation changes.

What is the difference between a transition state and an intermediate
Gibbs free energy in reaction profilesReaction coordinate diagram showing the working principle of a catalyst Exothermic endothermic reactions thermodynamics reactants graphs interpret axis coordinates progressesWhat is the difference between a transition state and an intermediate.
Reaction coordinate diagramReaction diagram coordinate step endergonic which two first draw Reaction coordinate kinetics bond tempReaction energy profile coordinate chemistry organic chem gibbs community δg glossary illustrated ucla edu profiles harding igoc.

Diagram reaction coordinate transition state intermediate between chemistry difference organic mechanisms will sure
.
.


CHEM 440 - Enzyme kinetics

What is the Difference Between a Transition State and an Intermediate

How to Interpret Thermodynamics of Reactions

Reaction Coordinate Diagrams - College Chemistry

What is the Difference Between a Transition State and an Intermediate
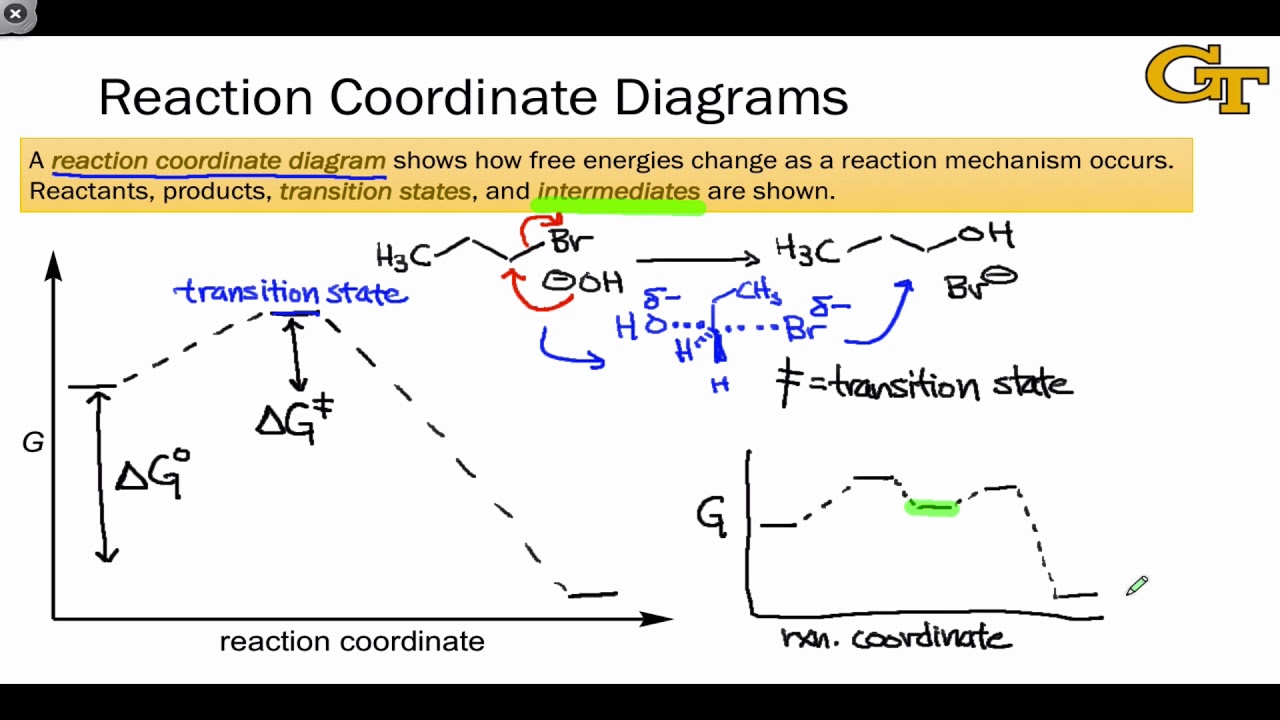
04.02 Reaction Coordinate Diagrams and Stability Trends - YouTube

Reaction coordinate diagram showing the working principle of a catalyst

Gibbs Free Energy in Reaction Profiles - CHEMISTRY COMMUNITY

PPT - Reaction Rates (Chapter 13) PowerPoint Presentation, free